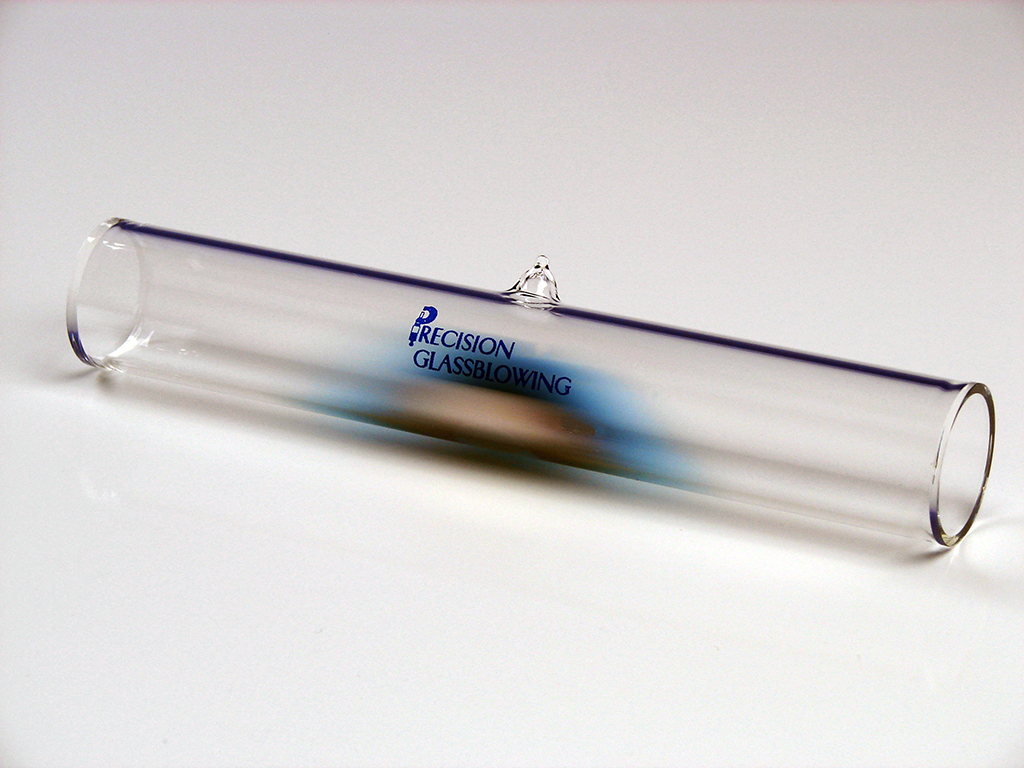
Vapor wavelength reference cells (VWR cells) are glass tube receptacles that contain vapor of specific atomic elements or molecular compounds. The vapor has a well-defined absorption spectrum, which means that it absorbs light at specific wavelengths.
These cells are commonly used in spectroscopic applications. The absorption spectrum helps to calibrate wavelength meters, tune diode laser calibration, stabilize laser frequencies, and measure the absorption of other materials.
Vapor Wavelength Reference Cells and Spectroscopy
VWR cells are critical for a variety of applications in spectroscopy. Calibration of wavelength meters helps to measure the wavelength of light. Vapor wavelength reference cells also help stabilize laser frequencies for optical communication and precision measurement. In addition, VWR cells are a key measurement tool to gauge the absorption of gases and liquids.

Tunable Diode Laser Calibration
A tunable diode laser is a type of laser that can change its output wavelength by adjusting the current or temperature. By comparing the output of the laser with the absorption spectrum of a vapor cell, one can calibrate the laser and ensure its accuracy and stability.
Shop Now

Stabilization of Laser Frequencies
A laser frequency is the number of cycles of light per second that a laser emits. Laser frequencies can drift over time due to environmental factors such as temperature, pressure, or humidity. By locking the laser frequency to a specific absorption peak of a vapor cell, one can stabilize the laser frequency and prevent drift.
Shop Now

Calibration of Wavelength Meters
A wavelength meter is an instrument that measures the wavelength of light. Wavelength meters can have errors or uncertainties due to numerous factors such as alignment, calibration, or resolution. By using a vapor cell as a reference standard, one can calibrate the wavelength meter and reduce errors or uncertainties.
Shop Now

Vapor Wavelength Reference Cell Design
The reason vapor wavelength reference cells are made from glass is because it is a transparent material. Transparency allows light to pass through the cell. A vapor wavelength reference cell consists of a glass tube with two windows at the ends, which allow light to pass through without significant loss or distortion.
Glass also has a low thermal expansion, which means that it does not change its shape or size much when heated or cooled. This helps maintain the integrity and alignment of the cell.
The tube can be made from borosilicate glass or quartz glass depending on the type of vapor and its operating temperature. It is baked and evacuated to remove any contaminants or impurities inside.
Using direct injection, diffusion, or sublimation, our cell is filled with gas or a mixture of gases containing the vapor of interest. The gas of interest is specific to the need. That is because its atoms or molecules, depending on the type of gas, absorb light at specific wavelengths. This absorption spectrum is what allows VWR cells to be used for calibration and other applications.
The tube is then sealed by melting the glass at the fill stem. The tube is helium leak checked to ensure the longevity of the cell.
Shop Now

A Matter of Gases
Vapor wavelength reference cells can be filled with gas. This gas can provide a suitable environment for the vapor to exist and interact with light. Gas allows the vapor to move evenly throughout the cell and to maintain constant pressure and temperature.
Gas also acts as a buffer that reduces collisions between the vapor atoms or molecules and other gas particles. This helps preserve the spectral features of the vapor.
The vapor can be an alkali metal (such as cesium, rubidium, sodium, or potassium), a halogen (such as iodine), or another element or compound (such as thallium, indium, or water). These vapors have characteristic absorption spectrums. This means they absorb light at certain wavelengths and not others. By measuring the absorption of light by the vapor, one determines the wavelength or frequency of the light source.
Shop Now

Types of Vapor Wavelength Reference Cells
There are several distinct types of VWR cells, each of which is filled with a different gas. The most common types of VWR cells are:
Rubidium (Rb) cells
Rb cells are filled with rubidium vapor, which has an absorption spectrum in the visible and near-infrared region. Rb cells are commonly used to calibrate wavelength meters and stabilize laser frequencies.
Cesium (Cs) Cells
Cs cells are filled with cesium vapor, which has an absorption spectrum in the visible and near-infrared region. Cs cells are also commonly used to calibrate wavelength meters and stabilize laser frequencies. Plus, they are widely used for atomic clocks and time standards
Iodine (I) Cells
I cells are filled with iodine vapor, which has an absorption spectrum in the visible and near-infrared region. I cells are commonly used to measure the absorption of other materials, such as gases and liquids. They are also for wavelength meter calibration and laser frequency stabilization.
Sodium (Na) Cells
Na cells are filled with sodium vapor, which has an absorption spectrum in the visible and near-infrared region. Na cells are less commonly used than Rb and Cs cells, but they can be used for similar applications. Sodium cells are sometimes with laser frequency stabilization and calibration.
Potassium (K) Cells
K cells contain potassium vapor, which has an absorption spectrum in the visible and near-infrared regions. K cells are less commonly used than Rb and Cs cells, but they can be used for similar applications.
Thallium (Tl) cells
Tl cells contain thallium vapor, which has an absorption spectrum in the visible and near-infrared regions. Tl cells are less common than Rb, Cs, Na, and K cells, but they work for similar applications.
Indium (In) cells
In cells contain indium vapor, which has an absorption spectrum in the visible and near-infrared regions. In cells are less common than Rb, Cs, Na, K, and Tl cells, but they also work for similar applications.
Isotopically Enriched Rubidium and Potassium Cells
Some VWR cells contain isotopically enriched rubidium or potassium. This means that the cells have only a specific isotope of the element. For example, this might be rubidium-85 or potassium-41.
Isotopically enriched cells are more stable than cells that contain a mixture of isotopes. Therefore, we prefer them for more accurate calibration and measurement.
Shop Now
Understanding Buffer Gases
Some VWR cells include buffer gases, such as helium, neon, argon, krypton, xenon, nitrogen, or ethane. That is because these gases help to stabilize the vapor pressure in the cell, which can improve the accuracy of calibration and measurement.
Buffer gases are gases that we add to the vapor wavelength reference cell along with the vapor of interest. However, they don’t have significant absorption spectra in the wavelength range of interest, so they don’t interfere with the measurement.
Buffer gases serve two main purposes:
• They increase the pressure inside the cell, which affects the density and temperature of the vapor. By adjusting the pressure, one can optimize the spectral features of the vapor for different applications.
• They reduce collisions between the vapor atoms or molecules and other gas particles, which can cause broadening or shifting of the spectral peaks. As a result of reducing collisions, one can preserve the spectral features of the vapor.
Shop Now
Types of Buffer Gases
Some of the common buffer gases in vapor wavelength reference cells are helium, neon, argon, krypton, xenon, nitrogen, and ethane.
The choice of buffer gas depends on factors such as:
- Type of vapor and its spectral properties
- Operating temperature and pressure of the cell
- Desired spectral resolution and accuracy
- Compatibility with the glass material
Why is that important? Because buffer gases help improve the performance and reliability of vapor wavelength reference cells. They can help achieve:
- Higher signal-to-noise ratio: By increasing the pressure inside the cell, the amount of light absorbed by the vapor increases, resulting in a higher signal-to-noise ratio.
- Narrower spectral linewidth: By reducing collisions inside the cell, spectral peaks broaden or shift.
Shop Now
The Importance of VWF Cells and Spectroscopy
Vapor wavelength reference cells are important because they provide reliable and precise standards for spectroscopic measurements. Spectroscopy is the study of how matter interacts with electromagnetic radiation, such as light.
Spectroscopy has many applications in science, engineering, medicine, industry, and other fields. For example, spectroscopy can:
- Identify chemical elements or compounds by their spectral signatures
- Analyze the composition or structure of materials by their spectral properties
- Detect trace amounts of substances by their spectral signals
- Monitor environmental conditions or changes by their spectral effects
- Measure physical quantities such as temperature, pressure, density, or velocity by their spectral variations
To perform these tasks accurately and reliably, one needs to have precise and stable sources and detectors of light. For that reason, VWR cells can help achieve this by providing well-defined and reproducible spectral features. These help to calibrate or stabilize the sources and detectors.
Shop Now

Precision Glassblowing’s VWR Cells
In summary, vapor wavelength reference cells are a valuable tool for a variety of spectroscopic applications. They calibrate wavelength meters, stabilize laser frequencies, and measure the absorption of other materials. Made from glass and filled with gas vapor, they are available in a variety of types. For example, these include Rb, Cs, I, Na, K, Tl, and In cells. Some VWR cells contain isotopically enriched rubidium or potassium. They may contain buffer gases to improve accuracy.
PGB Optical offers high-quality vapor wavelength reference cells and gas cells for spectroscopy. We carry the following vapor wavelength reference cells: Rubidium (Rb) cells, Cesium (Cs) cells, Iodine (I) cells, Sodium (Na) cells, Potassium (K) cells, Thallium (Tl) cells, or Indium (In) cells.
We also offer isotopically enriched Rubidium cells (85 and 87) and Potassium cells (41)*. The purity level of the natural elements in vapor cells is greater than 99%. After distillation, it reaches an even higher purity during the filling process. Upon request, we offer buffer gases like Helium, Neon, Argon, Krypton, Xenon, Nitrogen, and Ethane as well as paraffin coating and OTS coating.
*Due to the very high cost of Potassium 40 material, PGB Optical no longer offers reference cells filled with K40 vapor.
We inspect all vapor cells for helium leaks. Next, they bake under vacuum to the 10-8 Torr range before we fill them with the select element.
PGB Optical also offers gas cells filled with Acetylene, Hydrogen Cyanide (Isotope 12 and 13), Carbon Monoxide (Isotope 12 and 13), and Methane. All gases that we utilize in the manufacturing process have a purity level greater than 99%.
Reference cells are available in the following materials and configurations:
- Borosilicate with flat windows attached parallel or angled
- Quartz with flat, high-quality fused silica windows attached parallel or angled
- Quartz with wedged, high-quality fused silica windows attached parallel or angled
- Quartz with flat, anti-reflective (AR) coated high-quality fused silica windows attached parallel or angled
Shop Now